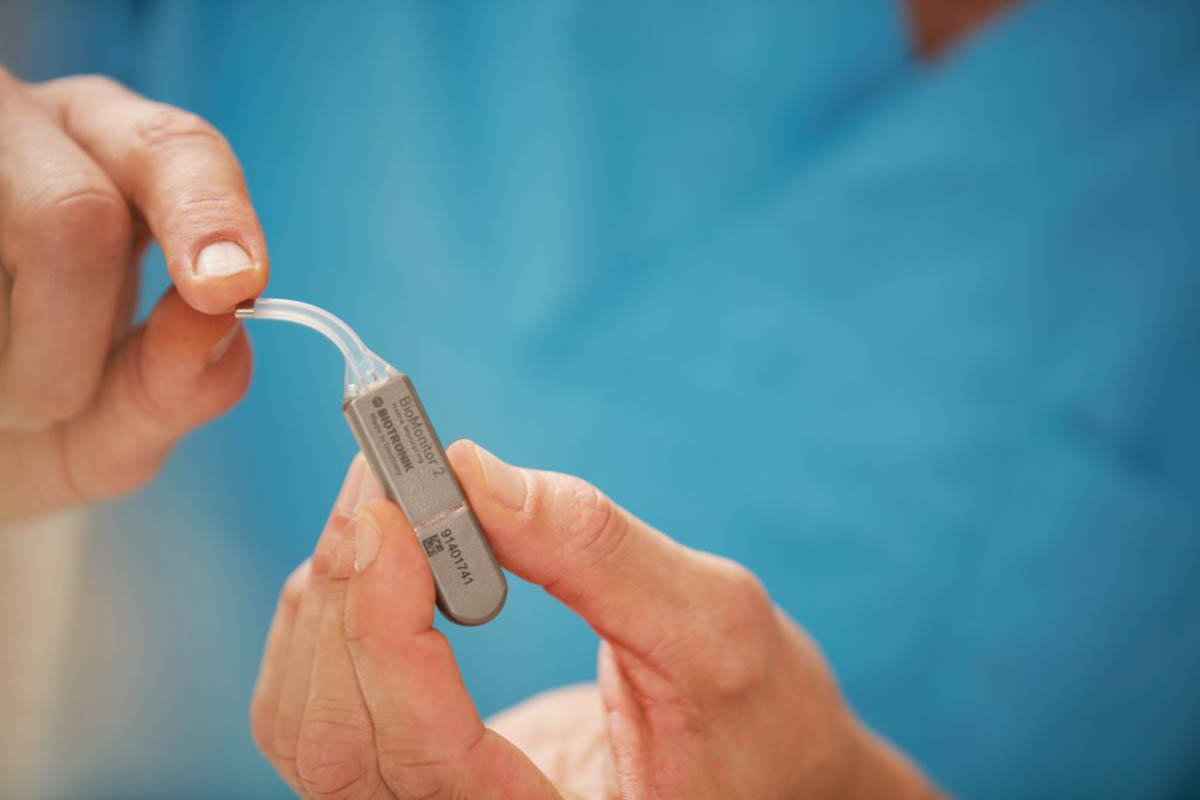
Biotronik has declared clearance and approval by the FDA of an important Data Sensor in the latest Biomonitor IIIm injectable cardiac monitor (ICM). The sensor detects body temperature changes that may also be associated with fever.
The connection between vital signs, such as body temperature and cardiac arrhythmia, and health problems has been long known. Temperature increases, in particular, can cause a rise in heart rates and, as a result, a reduction in the patient’s activity level.
With the Data Sensor, doctors can access comprehensive updates on critical data to assist them in remotely tracking patient’s overall health and condition.
This technology is a step forward for injectable cardiac monitors. Luigi Di Biase, an expert at Montefiore Health System, said that it gives him and his patients relief to know that we will be able to track for early symptoms of possibly fatal cardiac arrhythmias and infections.
Also, Biotronik claimed that this device has a unique design that was developed with practicality in mind. It delivers highly-accurate results and reliable detection ability. And, more importantly, it can be used for constant monitoring for up to 5.5 years.